Ion signaling in developing cells and tissues
We measure and control ionic activities in live, developing cells and tissues to understand how biology might do the same.
Contact Bill for more information.
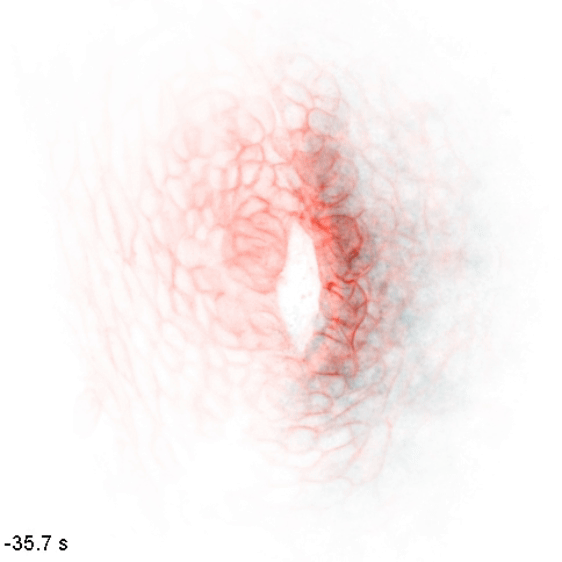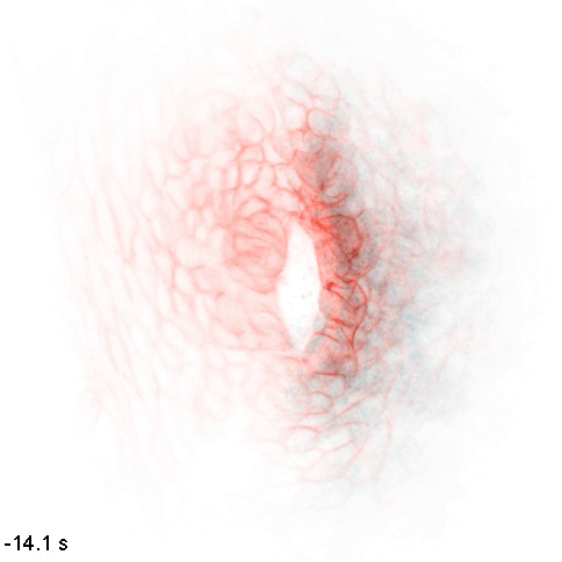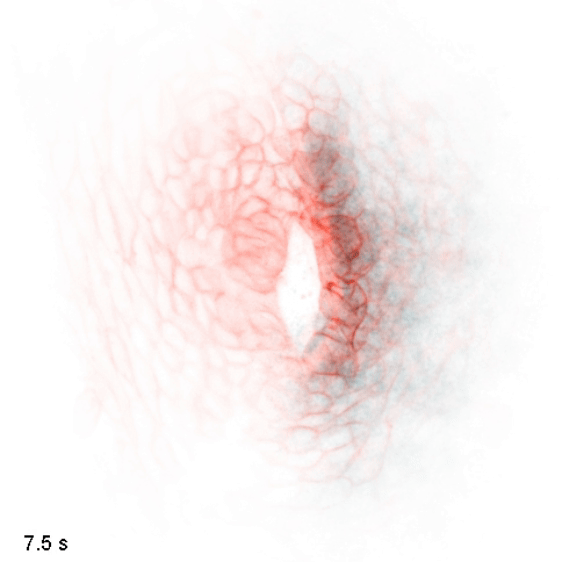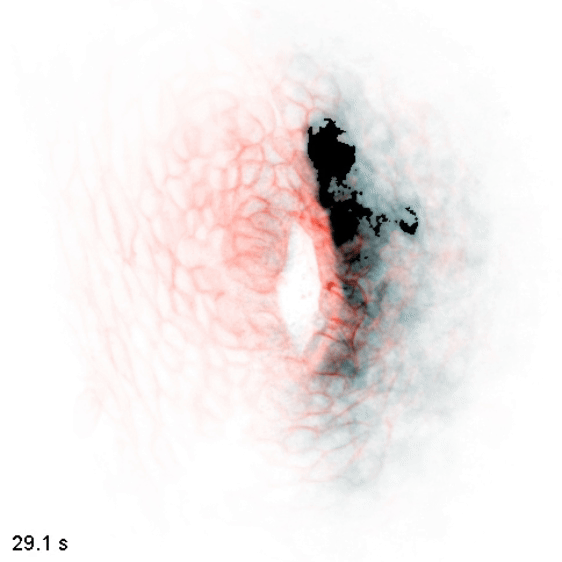
The wiring diagram of electrical charges in eukaryotic cells and tissues
Monoatomic ions (Na+, K+, Ca2+, Cl-, etc.) are some of the simplest and most abundant chemical species in the biological milieu, but the regulation of their distribution is complex: the human genome contains up to 2000 genes thought to encode components of ion channels and transporters. We aim to map flows of intracellular and extracellular ions, and determine how these can direct cellular decisions on growth, motion, and differentiation. We study these behaviors in the context of embryonic development, where naïve tissues must alter their ion physiology along stereotyped trajectories to form specialized organs such as the heart, brain, and kidney. To do so, we use and develop biosensors and optogenetic tools to nondestructively measure and control these processes, along with other molecular, genetic, and modeling approaches. We use the zebrafish embryo as a model to understand cellular computation in a natural context and to observe emergent tissue-level phenomena, and cultured cells as a model to get simplified access to the underlying biochemistry and subcellular biophysics.
Projects
In embryonic development, organs need to build their size and shape, as well as their function. How do form and function talk to each other? We know that the mechanical motion of the heart during its pumping is important for determining its structure, but could the upstream electrical activity be important too? We made tools for looking at and controlling this electrical activity in developing embryos, and we’re now using them to find out.
The electrical action potential is perhaps the biological phenomenon most similar to human-designed digital information processing systems. It just makes sense that neurons and muscles would go to great lengths to encode binary information using action potentials. But some of the molecules they use for this are also found in “non-excitable” cell types that don’t fire action potentials, except their dominant operating mode is probably analog instead of digital. What are the rules of this analog processing, and how are physical components put together into a machine that performs these computations? We are trying to learn the rules by combining our methods for controlling ion physiology with tools to measure signaling outputs that might represent this information inside non-excitable cells and turn it into other biological behaviors.
Your body goes to great lengths to control intracellular and extracellular ion content. This is needed for organs like the brain, heart, and pancreas to work the way they expect to, and it is one of the main jobs of your kidneys and bladder. But embryos at the earliest stages of development don’t have kidneys and bladders, and different species grow up in environments surrounded by very different ion concentrations. How do they manage, and does this matter for development?
How does the electrical activity of the heartbeat direct its development?
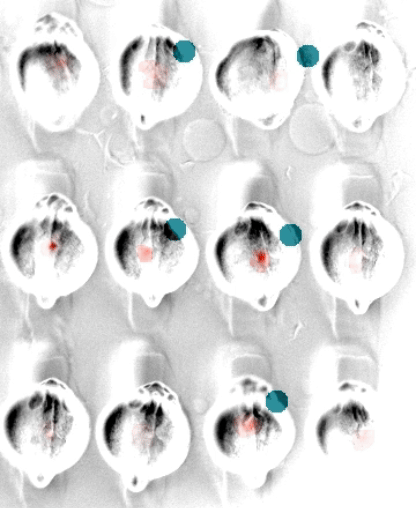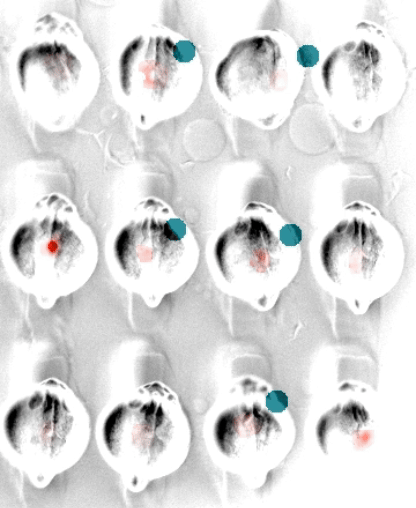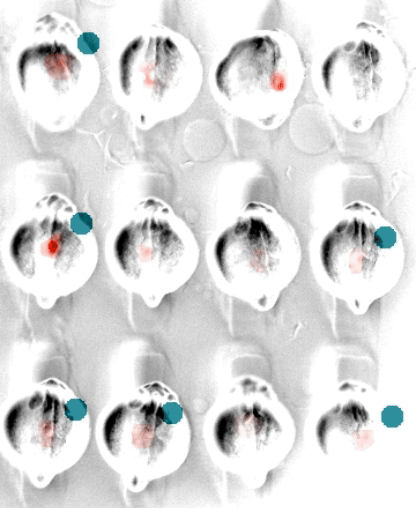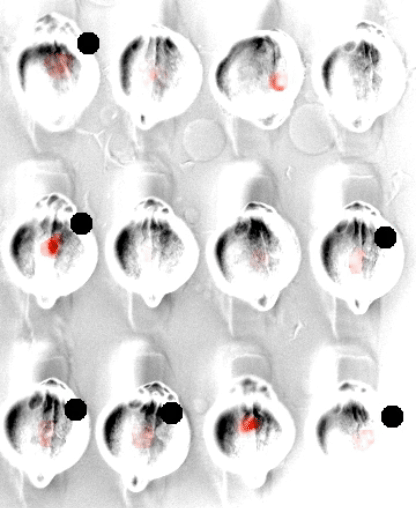
In embryonic development, organ-scale form and function must emerge in lockstep. Perhaps nowhere is this more evident than in the embryonic heart, which must in concert form its chambers, connect to the circulatory and nervous systems, and establish the autonomous electrical activity and contraction essential for life in all vertebrates. Physiology and morphogenesis are likely coordinated by bidirectional sensing relationships and mutual feedback, but the rules by which cells measure organ-scale physiological activity and use it to make developmental decisions are poorly defined.
We are investigating how the ionic fluxes driving cardiac electrical activity, upstream of contraction, can direct morphogenesis and cardiomyocyte specification. Using frontier optogenetic tools and live cell biosensors, we have established the basic electrophysiological events underlying the initiation of spontaneous action potentials and calcium dynamics in the developing zebrafish heart. We are now extending these tools to directly control specific pools of subcellular calcium and image calcium-dependent signaling activities, with the goal of exploring how calcium signaling changes over time and space in the hearts of intact, developing embryos. We suspect that other aspects of electrical function, aside from calcium elevation, may also have instructive roles in cardiac development.
We are also interested in the molecular mechanisms underlying the early establishment and maturation of electrical activity in the heart - whether the first “sparks of life” are driven by production of particular ion channels and transporters, and/or posttranslational modulation of their activity.
How do biological processes sense ion physiology in non-excitable cells?

Neurons, myocytes, and other spiking (“excitable”) cells have an electrophysiology adapted to produce rapid electrical events (action potentials). These serve as well-defined “packets” of information that are usually transduced into other biological function through downstream intracellular calcium elevation. Yet all cells, not just spiking ones, tightly regulate the transport of ions across their membranes. In addition to calcium entry, myriad biophysical effects induced by ion transport, such as electric fields (membrane potential), osmotic stress, and intermolecular interactions required for protein folding, probably have profound implications for cellular function. We have little understanding of the molecular mechanisms with which cells might interpret these features, and whether they are diverse or conserved across organ systems and across the tree of life.
We are establishing the biophysical and molecular grammar of ion sensing in non-excitable cells. We are combining light- and ligand-gated ion channels/pumps with tools for measuring the dynamics of cell morphology and signaling in closed-loop “smart microscopy” pipelines, to systematically define the input-output relationships and control architectures between ion fluxes and other cellular processes. We are also developing imaging and mass spectrometry-based assays to visualize localization and phosphorylation of proteins upon ionic perturbation, to search for the underlying molecular and biophysical mechanisms that determine downstream signal propagation. We further hope to understand how these relationships vary across cell context and type, noting that even specialized spiking cell types start off as non-excitable in development.
How do embryos develop in diverse ionic environments?
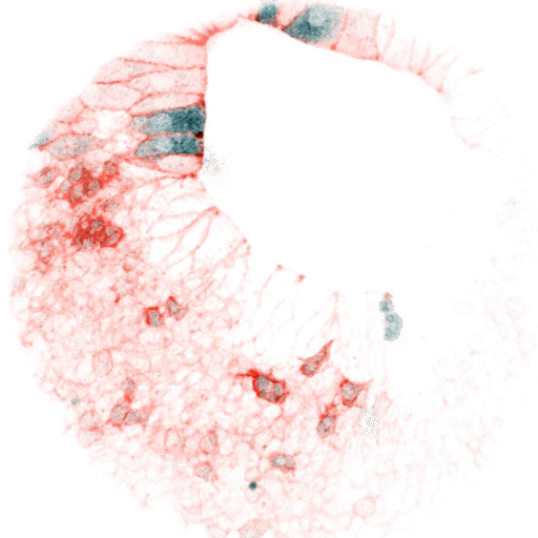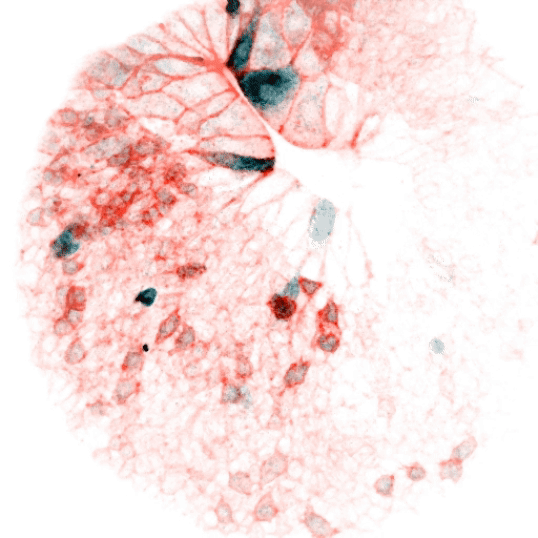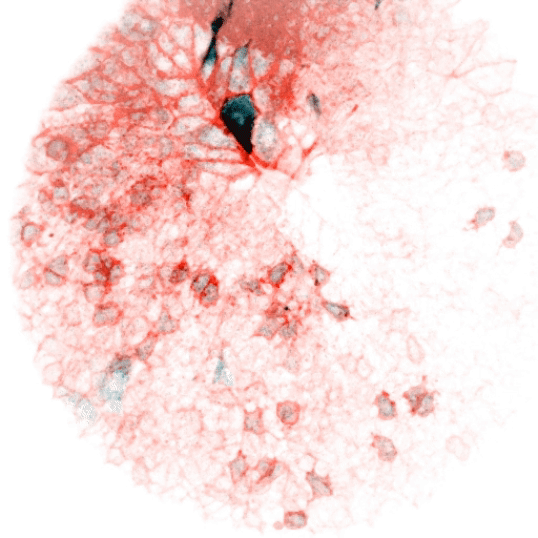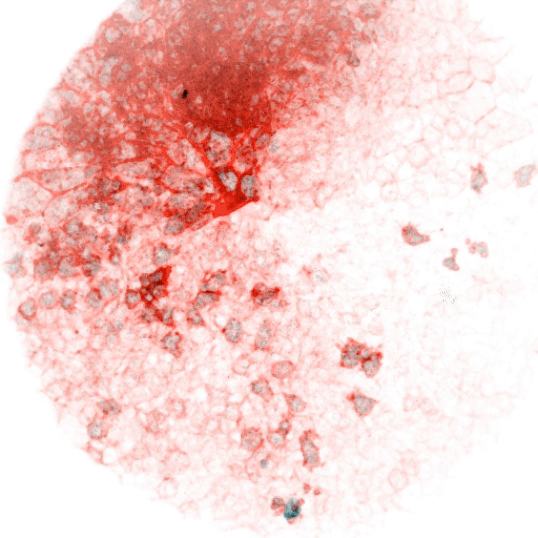
Vertebrate animals live in diverse surroundings, but their intracellular and extracellular ion concentrations are remarkably similar due to organismal scale barrier function and osmoregulation through organs such as kidneys, gills, and sweat glands. But fertilized eggs have no such capabilities and are ejected into drastically different environments (e.g. fresh versus salt water). This raises some easily stated, but unsolved questions: how does a zygote in fresh water (e.g. the zebrafish) not rupture in its hypotonic environment, and how does one in salt water not collapse? At what point are more “adult-like” extracellular conditions established, and how does the ion physiology of cells rewire accordingly? We reason that the cellular composition and function of channels and transporters must change dramatically in the early stages of development. We are interested in the nature of these differences over developmental time and between species, how they are regulated, and whether they serve as triggers or checkpoints for other developmental events. These processes are likely relevant not only to physiology, but to evolutionary transitions from unicellularity to multicellularity and between different ecological niches. They may also be important to our understanding of adaptability and fitness of aquatic organisms as the Earth’s watersheds are reshaped by climate change.
Technologies
All-optical physiology
Using sensors of physiology and signaling and optogenetic actuators to measure and control biological processes with light
Synthetic biology and protein engineering
Novel tools to measure, record, and actuate biology in vivo, and spatiotemporal control of their function
In toto imaging
Capturing dynamics at cellular resolution across a whole embryo or tissue as it develops
Automated and AI image analysis
Extracting biological insight from complex datasets with scale and rigor, and formalizing their relationship with physical models
Patch clamp electrophysiology
Classic approaches to understand the biophysics of ion channels and the functional identity of cells
Come help us learn:
If you are either a prospective lab member or collaborator, we have projects that would have great synergy with these skills.
Genetic screens and functional ‘omics
Single-molecule techniques
Stem cell and organoid culture
De novo protein design
Scaling science with AI